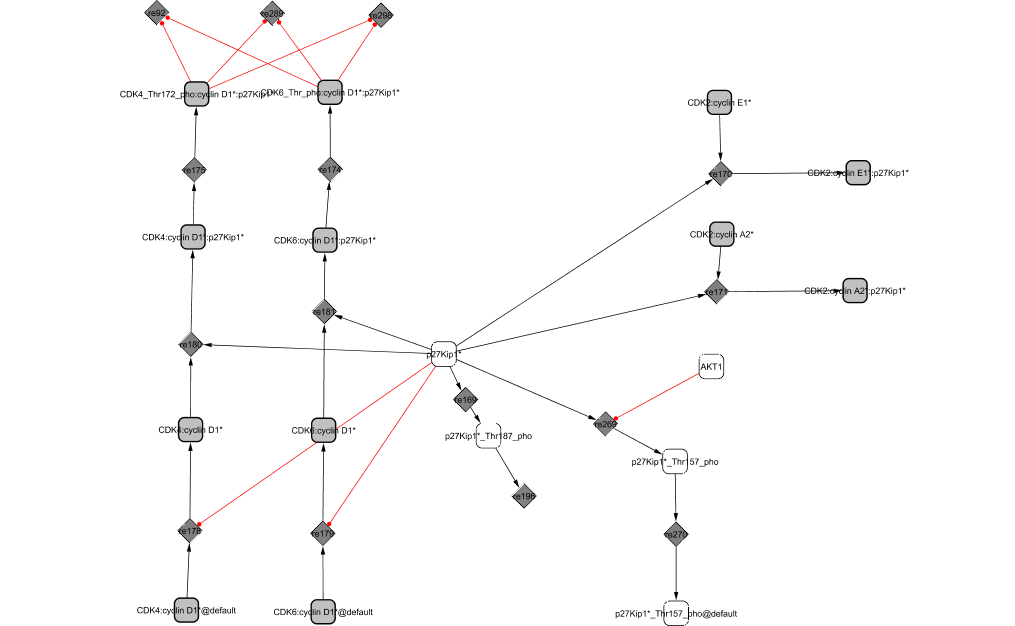
p27KIP1 is a cyclin-dependent kinase inhibitor. It binds to CycD1/CDK4,6, CycE1/CDK2 and CycA2/CDK2 to form trimers. However, p27KIP1 seems to have an activating role in the case of CycD1/CDK4,6 association: it favours the binding of the complex and directs its accumulation to the nucleus, as already mentioned in module CycD1/CDK4,6. In experiments, p27KIP1 has been found to co-immunoprecipitate with the active complex. On the contrary, when bound to CycE1/CDK2 and CycA2/CDK2, p27KIP1 acts as a stoichiometric inhibitor (Sherr and Roberts, 1999). Initially in the nucleus, p27KIP1 is phosphorylated by Akt1, which induces its translocation to the cytoplasm where it can no longer interact with the CDK complexes. Towards the end of S phase, CycE1/CDK2 phosphorylates it at Thr-187 which targets it for degradation (see module CycE1/CDK2).
p21CIP1 is regulated in a similar fashion, except for its phosphorylation and degradation mediated by CycE1/CDK2. It also binds to CycD1/CDK4,6 and stabilizes the complex whereas it inhibits CycE1/CDK2 and CycA2/CDK2. p21CIP1 seems to inhibit CycB1/CDC2 as well (Child and Mann, 2006).

|

