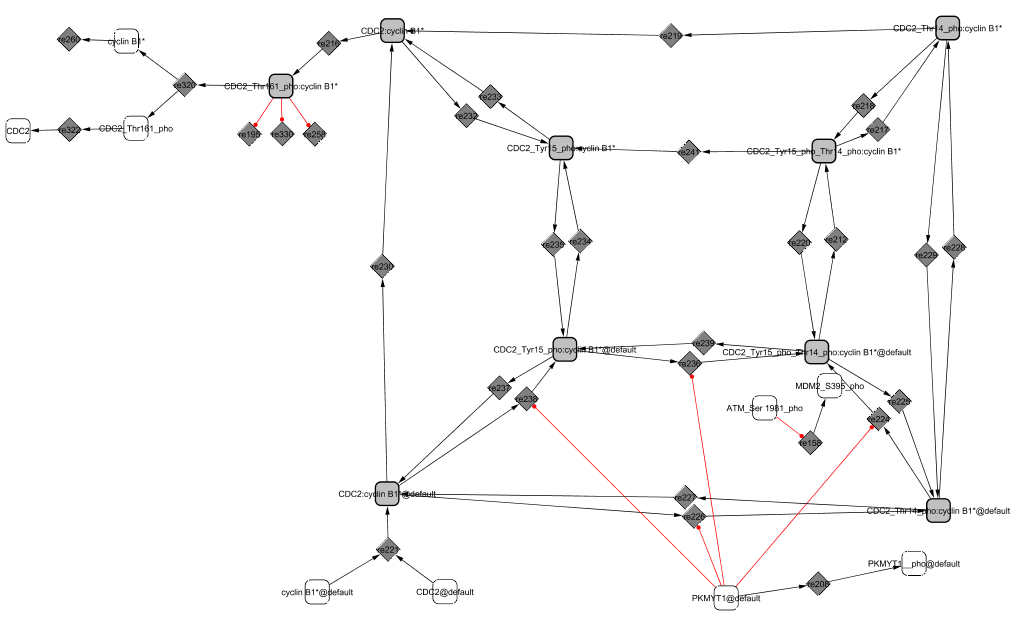
The last cyclin intervening in the cycle controls entry and exit of mitosis. CycB1/CDC2 activates at the G2/M transition and precipitates the cell in M phase.
CDC2 and cyclin B1 bind in the cytoplasm. Only when cyclin B1 is in complex with CDC2 can it be phosphorylated at Thr-14 and Tyr-15 and kept inactive by the protein kinase PKMYT1 (Liu et al., 1997; Liu et al., 2003; Vermeulen et al., 2003). The dephosphorylation and activation of the CDK complex is controlled by the phosphatase CDC25C (Vermeulen et al., 2003). The complex is transported in the nucleus at prophase (Pines and Hunter, 1994). In the nucleus, the phosphorylation and inactivation of the CycB1/CDC2 complex is insured by the kinase WEE1 and the dephosphorylation and activation by the nuclear form of CDC25C (Heald et al., 1993). To be fully active, CycB1/CDC2 needs to be phosphorylated at Thr-161 by CAK (CycH/CDK7/MNAT1) (Coqueret, 2002). CycB1/CDC2 can then phosphorylate both CDC25C and WEE1 creating a positive feedback with these proteins by activating CDC25C (its activator) more and inactivating WEE1 (its inhibitor). The complex eventually dissociates and cyclin B1 is targeted for degradation by CDC20/APC (Fung and Poon, 2005).
The different activities of the cyclins orchestrate the events of the cell cycle. They need to be regulated through the binding to stoichiometric inhibitors (see modules p16INK4a / p15INK4b and p27KIP1 / p21CIP1), through phosphorylation (see module CDC25C / WEE1) or through degradation of the cyclin partners (see APC module).
|

